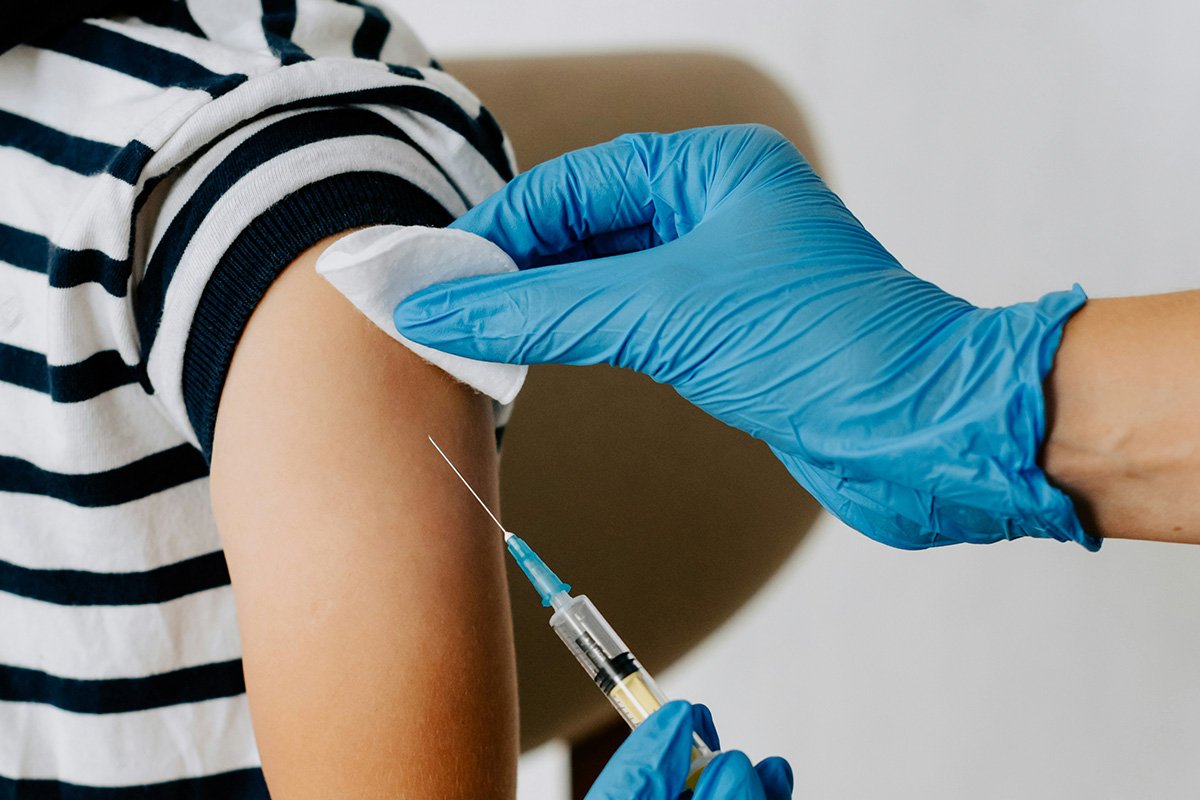
"The federal government's latest guidelines for COVID-19 vaccines make it difficult to know who, exactly, will be able to access shots this fall. While Health and Human Services Secretary Robert F. Kennedy Jr. and some of his staff claim anyone will be able to access a shot in consultation with their doctor, medical groups are warning that the new guidance will impact a broad swath of people, including postpartum people and healthy children."
"Actions by the Food and Drug Administration last week mean that none of the COVID-19 vaccines that are slated to be on the U.S. market this fall will have an emergency use authorization that had allowed their quick (yet still rigorously tested) approval at the height of the pandemic. The removal of this designation means the drug company Pfizer will no longer offer COVID-19 vaccines to very young children, limiting parents' brand options and potentially impacting supply."
Federal guidelines for fall COVID-19 vaccines create uncertainty about who can access shots, with HHS Secretary Robert F. Kennedy Jr. and staff saying anyone can obtain a dose in consultation with a doctor, while medical organizations warn the guidance will affect many people, including postpartum individuals and healthy children. Pediatricians report significant constraints for children and young adults. No-cost vaccine coverage under insurance may not be determined until a vaccine advisory panel meets in mid-September. FDA actions removed emergency use authorizations for upcoming vaccines, prompting Pfizer to stop offering vaccines to very young children and limiting brand and supply options. Moderna, Pfizer, and Novavax released eligibility details.
Read at Non Profit News | Nonprofit Quarterly
Unable to calculate read time
Collection
[
|
...
]