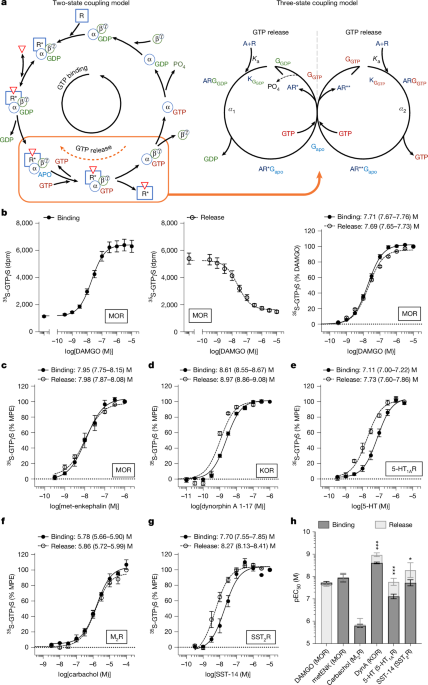
"Heterotrimeric G proteins transduce information to intracellular partners by modulating GTP binding and hydrolysis3. Through their interaction with G-protein-coupled receptors (GPCRs) and effectors, G proteins provide the transducer function that is necessary for the conveyance of extracellular information4,5. Heterotrimeric G proteins consist of an α subunit bound to a β and γ subunit dimer; they remain a trimer while the α subunit is bound to GDP6."
"One such study examined the kinetics of the release of 35S-GTPγS in cells expressing the mu opioid receptor (MOR) and found that the rate of release of nucleotide was increased as a function of a single saturating concentration of agonist and that partial and full agonists maintained their rank order efficacy in both exchange reactions13 ( 35S-GTPγS binding and 35 S-GTPγS release)."
Heterotrimeric G proteins transduce signals by modulating GTP binding and hydrolysis through interactions with GPCRs and effectors. G proteins are composed of an α subunit associated with a βγ dimer and remain a trimer when the α subunit is GDP-bound. GPCRs undergo conformational changes that catalyse reactions with Gα, shifting affinity from GDP-bound states to favour GDP release and GTP binding, functioning as guanine-nucleotide-exchange factors. Observations indicate that receptors can also promote GTP release from Gα, demonstrated using nonhydrolysable GTP analogues and kinetic studies showing agonist-dependent nucleotide dissociation and preserved agonist efficacy order.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]