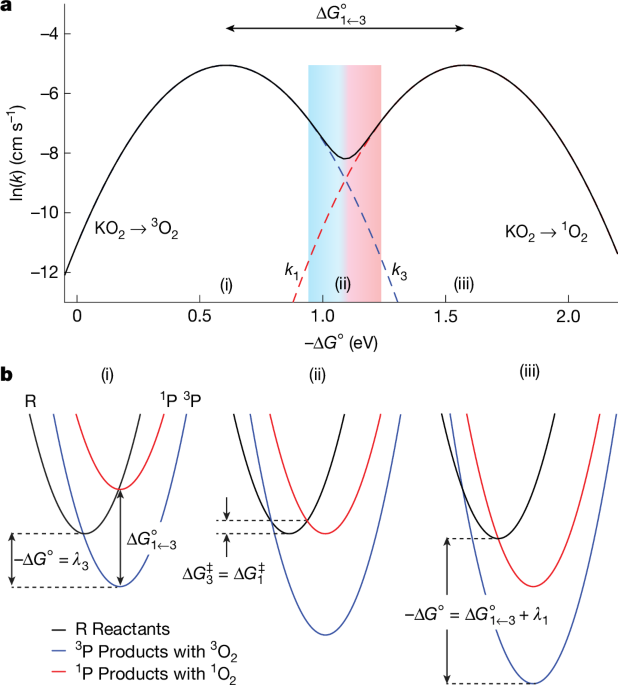
"More than half a century after the discovery that singlet oxygen forms from superoxide8, what governs the evolution of singlet or triplet oxygen under many conditions relevant to life and human-made oxygen redox systems remains unknown. Oxygen redox chemistry is crucial to life1 and encompasses some of the most fundamental and widespread chemical reactions, including those in batteries2,3,4,9, fuel cells and electrocatalysis10, and organic chemistry11."
"Whereas 3O 2 is relatively unreactive, 1O 2 is highly reactive with most organic matter11,12. This is readily evidenced in metal-ion batteries6,7 and metal-O 2 batteries3,4, in which 1O 2 is the primary source of degradation, causing decomposition of organic electrolytes and conductive carbon additives, which ultimately degrades the overall device function. Moreover, 1O 2 and (su)peroxide are well-known reactive oxygen species in biological systems and are involved in several processes from signalling to cell damage1,5."
More than half a century after the discovery that singlet oxygen forms from superoxide, what governs the evolution of singlet or triplet oxygen under many conditions relevant to life and human-made oxygen redox systems remains unknown. Oxygen redox chemistry spans oxidation states from dioxygen (0) to oxide (-2), with intermediate states such as superoxide (−½) and peroxide (−1). Superoxide is pivotal in oxygen reduction and evolution because it is closest to dioxygen. Triplet oxygen (3O2) is relatively unreactive, whereas singlet oxygen (1O2) is highly reactive and causes decomposition of organic electrolytes and conductive carbon in batteries and contributes to signalling and cell damage in biological systems. Superoxide disproportionation in protic and certain aprotic environments produces O2 and peroxide and yields either 3O2 or 1O2.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]