"Historically, ER sheets have been considered the predominant site of secretome mRNA translation, largely owing to their enrichment in membrane-bound polysomes5. Yet, recent reconstructions from diverse mammalian cell types reveal that ribosomes, including polysome-associated and monosome-bound forms, are distributed across nearly all ER morphologies, encompassing sheets, tubules and tubule-tubule junctions6. Notably, a substantial subset of ER-bound ribosomes corresponds to non-translating subunits, particularly the 60S7,8, suggesting that ribosome association is not synonymous with active elongation and may reflect regulatory or pre-initiation states."
"Definitive mapping of translation sites through Sec translocon components remains technically intractable because genetic tagging of core constituents such as Sec61α or TRAP often perturbs translocation activity, and immunolabelling approaches are hindered by steric inaccessibility of lumenal or membrane-embedded epitopes9."
"We demonstrate that active secretome mRNA translation is concentrated at ER junctions that are enriched in the structural protein lunapark (LNPK) and positioned adjacent to lysosomes. Loss of LNPK selectively reduces ribosome occupancy and translation efficiency of secretome mRNAs at these junctions by impairing eIF2-dependent initiation, an effect that can be rescued by the integrated stress response (ISR) inhibitor ISRIB."
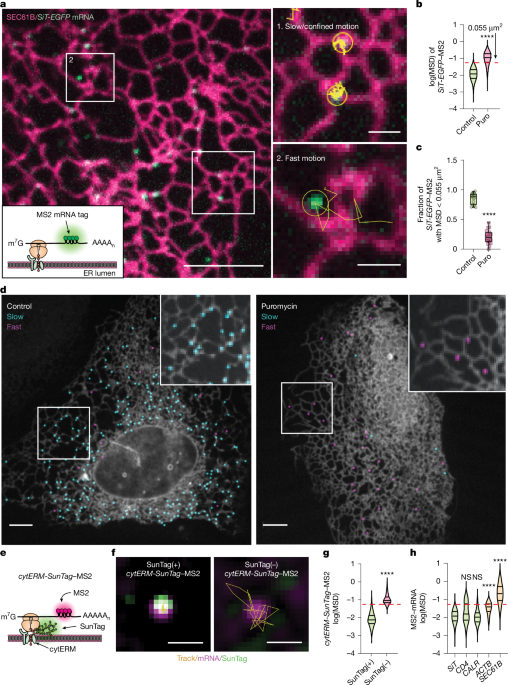
ER ribosomes, including polysomes and monosomes, distribute across sheets, tubules and junctions despite ER sheets' polysome enrichment; many ER-bound ribosomes are non-translating 60S subunits. Tagging Sec translocon components perturbs translocation and immunolabelling is limited by steric inaccessibility, making translation-site mapping difficult. Live-cell tracking reveals active secretome translation concentrates at LNPK-enriched ER junctions adjacent to lysosomes. LNPK loss reduces ribosome occupancy and translation efficiency at these junctions by impairing eIF2-dependent initiation, and ISRIB rescues the defect. Translation at lysosome-proximal ER increases during amino acid starvation and decreases with lysosomal pH neutralization.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]