""In a perfect world, every cancer patient should get to be part of a clinical trial if they want to be," says Lola Rahib, an independent biomedical researcher and cancer patient advocate. This emphasizes the importance of decisions around which clinical trials to run and support, particularly regarding prioritizing cancer types and studying diverse patient demographics."
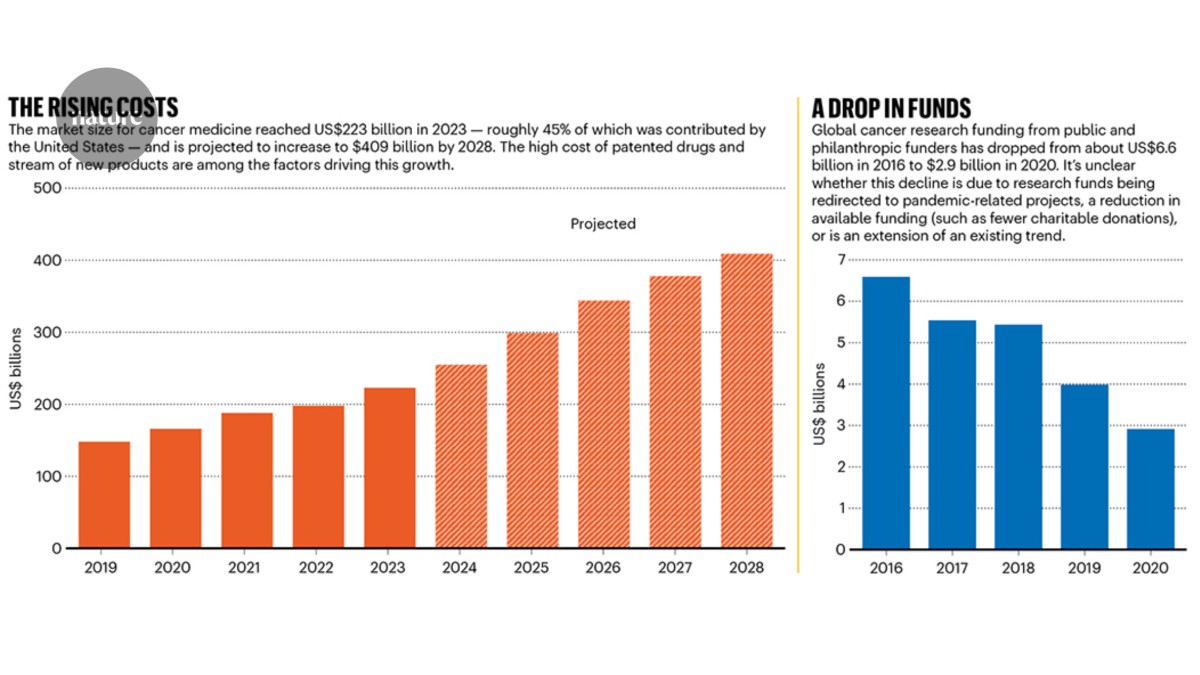
"The market size for oncology drugs is expected to reach US$409 billion by 2028, up from $223 billion last year, according to IQVIA Institute. This increase reflects the large volume of patented medications and the number of new cancer treatments introduced in the last five years."
"Emerging biopharmaceutical companies, defined as those with less than $500 million in sales annually, accounted for 60% of oncology trials in 2023. This is a significant increase from 33% in 2014, highlighting the agility and impact of smaller companies in the cancer clinical trial landscape."
Cancer is a major global health issue, responsible for approximately 15% of deaths worldwide in 2021 and projected to see 28 million new diagnoses annually by 2040. The rising burden necessitates rapid advancements in diagnosis and treatment, with clinical trials playing a crucial role. Lola Rahib advocates for better access to trials while recognizing the complexities of prioritizing cancer types and patient demographics. As oncology drug costs soar, emerging biopharmaceutical companies are increasingly influential, now leading a significant portion of clinical trials, indicating a shift towards innovation in cancer treatment.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]