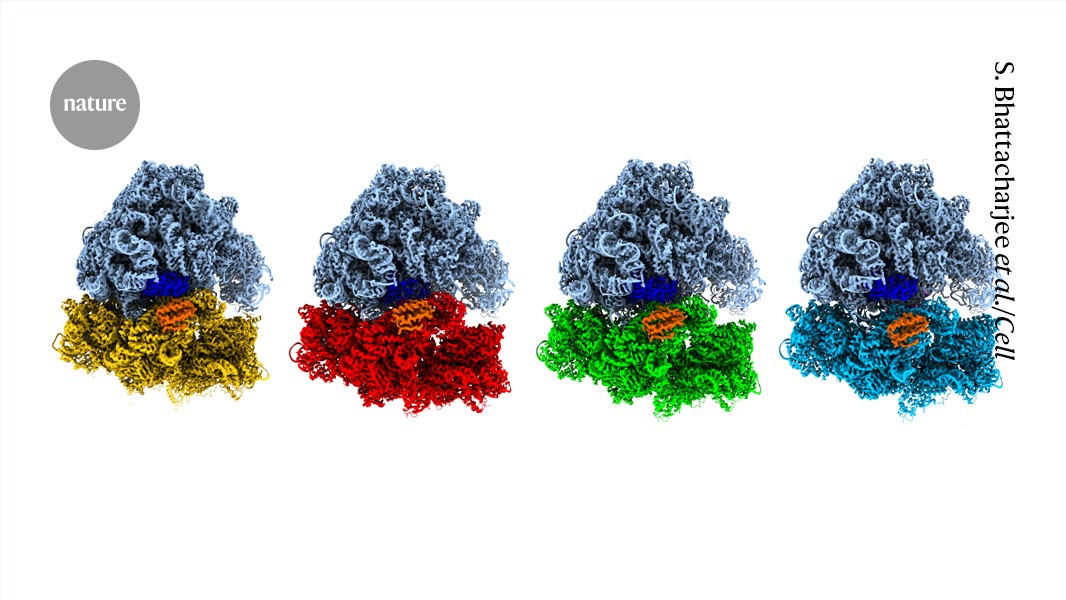
"Working on that timescale has long stymied biologists, but the rapid evolution of time-resolved cryo-electron microscopy (TR cryo-EM) over the past several years has made it possible to reconstruct dynamic processes with near-atomic detail."
"Beyond fundamental biological insights, a deeper understanding of protein dynamics could guide the development of more effective medicines and boost protein-engineering efforts."
"Time-resolved cryo-EM could be the natural choice - or only option - to capture [protein] conformations that could be potentially better targeted by drugs."
"Nature is under no obligation to be simple, cautions Radoslav Enchev, a biochemist at the Francis Crick Institute."
The article discusses the rapid advancements in time-resolved cryo-electron microscopy (TR cryo-EM), which allows scientists to capture the dynamic motions of proteins essential for understanding their functions. Traditional methods struggled with the timescale of protein dynamics, but TR cryo-EM enables near-atomic detail insights. This evolving technique holds potential not only for fundamental biology but also for drug discovery and protein engineering. However, its niche nature, requiring specialized skills and equipment, presents challenges for broader adoption among laboratories, potentially slowing progress in the field.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]